Rifaximin, a minimally absorbed antibiotic, has become a cornerstone therapy for several gastrointestinal disorders, including irritable bowel syndrome with diarrhea (IBS-D) and hepatic encephalopathy. Despite its established use, access to rifaximin varies significantly across global markets, with cost barriers posing challenges in some regions. In contrast, in many Asian countries, the same medication is available at a fraction of the price, raising important questions about pharmaceutical pricing practices and access equity.
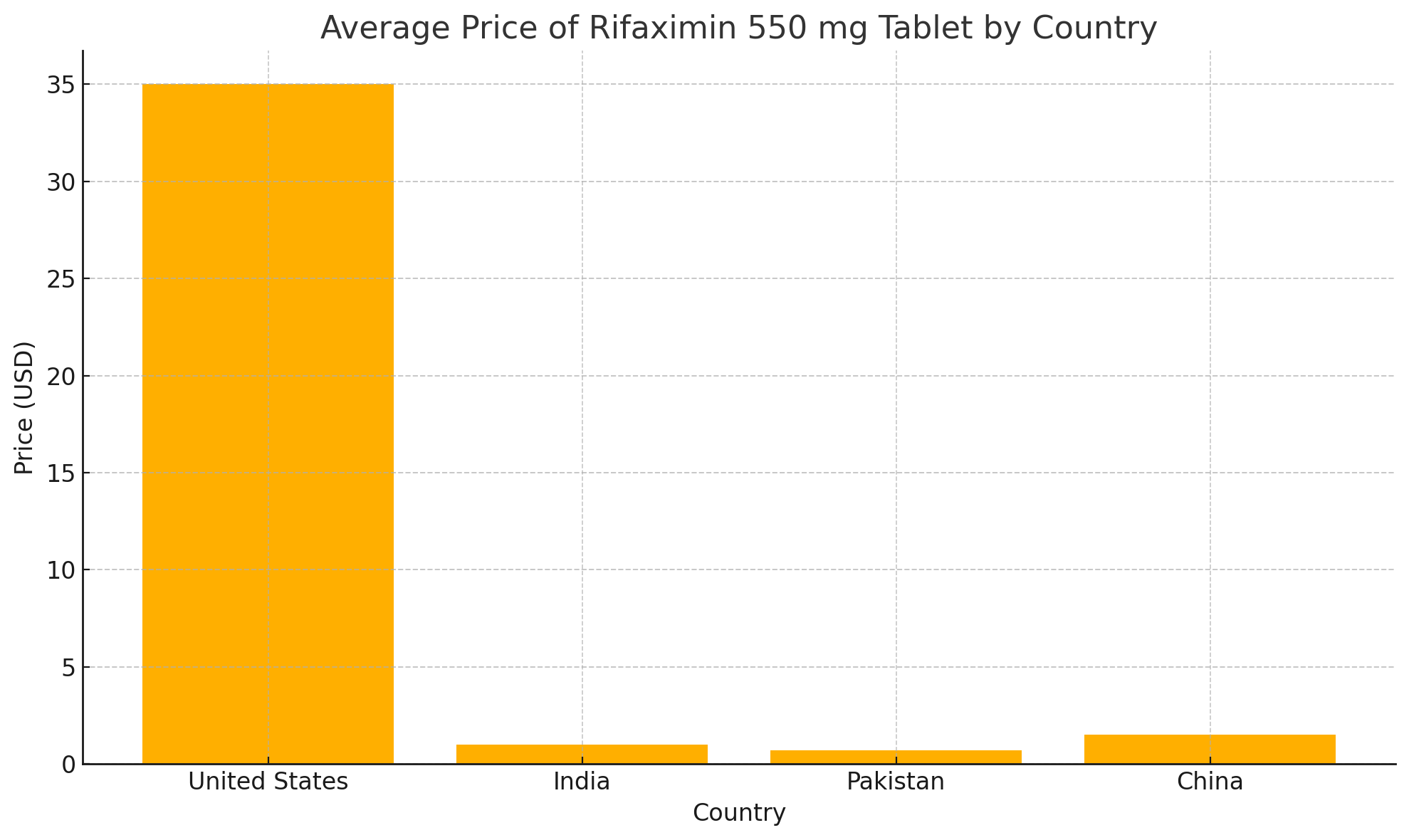
Using publicly available pharmacy databases, we compared the price of rifaximin 550 mg tablets across different markets. In the U.S., the average retail price for a single tablet was approximately $30 to $40, as listed on GoodRx. In comparison, prices in India, Pakistan, and China were significantly lower, averaging between $0.70 to $1.50 per tablet (Table 1). These findings highlight a price difference exceeding 95 percent between certain high-income and lower-income markets.
Several factors contribute to this disparity. In the U.S., rifaximin remains protected by multiple patents listed in the FDA’s Orange Book, delaying the introduction of generics until at least 2029. In contrast, many Asian countries implement pharmaceutical price regulations and allow earlier generic market entry, thereby reducing costs for patients.
The clinical implications of this pricing gap are substantial. High out-of-pocket costs may deter the appropriate use of rifaximin, potentially affecting outcomes in chronic conditions. Addressing these disparities through policy reforms that promote competition and price transparency could improve patient access to essential therapies.
In patients with irritable bowel syndrome with diarrhea (IBS-D), rifaximin provides a non-systemic, gut-directed treatment that has demonstrated efficacy in alleviating symptoms such as bloating and altered bowel habits. Given its well-established role in IBS-D management, equitable access to rifaximin becomes particularly important. In settings where medication cost may influence prescribing patterns or patient adherence, disparities in pricing can inadvertently limit clinical utility. Broader availability at an affordable cost could enhance symptom control, reduce the burden of disease, and allow clinicians to optimize care with confidence in both efficacy and tolerability.
In an era increasingly focused on health care value and equity, global pricing discrepancies warrant critical attention. Bridging the gap for medications such as rifaximin may serve as a model for broader pharmaceutical policy reforms aimed at improving affordability and access worldwide.
Jai Kumar is an internal medicine resident. Brian Nohomovich and Leonid Shamban are gastroenterologists.




















![AI censorship threatens the lifeline of caregiver support [PODCAST]](https://kevinmd.com/wp-content/uploads/Design-2-190x100.jpg)
